DETERMINATION OF BICARBONATE AND CARBONATE IN CONSUMER PRODUCTS
PRINCIPLES OF OPERATION
Samples are heated to 150˚C to release CO2 from the bicarbonate portion of the sample. A carbon dioxide-free carrier gas sweeps the evolved CO2 from the heated reaction flask into the CO2 Coulometer. The CO2 Coulometer automatically titrates the evolved CO2. After the CO2 from the bicarbonate has been titrated, the sample is then treated with acid to release CO2 from the carbonate portion of the sample. This CO2 is then swept into the coulometer and titrated.
PROCEDURE
- Assemble and prepare the components for operation as described in the Instruction Manuals.
- Determine the blank and run a standard to confirm proper operation of the complete system.
- Charge the sample tube with a known weight of sample and attach the tube to the apparatus. Sample size ideally should be selected to contain 1000ug – 3000ug carbon.
- Allow approximately one minute for the system to purge itself of atmospheric CO2 after attaching the sample tube.
- Move the sample tube into position in the heater block and start the coulometer.
- When all of the CO2 is evolved and titrated (recognized by a stable coulometer display) record the display value and calculate the results. 1
- Introduce acid into the reaction flask, approximately 10ml, and start the coulometer.
- When all of the CO2 is evolved and titrated from this portion of the analysis record the display value and calculate the results. 1
1 – Endpoint determination and result calculations are performed automatically based on user selectable setting entered into the CM5017 CO2 Coulometer.
RESULTS
When samples contain over 1000ug C, the titration accuracy is better than +/-0.15% relative. Overall accuracy is typically +/-0.3% relative. When sample availability limits the amount of CO2 evolved to small amounts, the accuracy is generally better than 1ug C.
Total analysis times are typically 15 to 20 minutes. For some samples the reaction time with either the heat or the acid is very slow, thus extending the analysis time.
A major advantage of the CO2 Coulometer is that no chemical calibration is required, also the analysis completion can be seen, avoiding low results due to incomplete analysis times or wasted time due to overly long analysis times. Other advantages include the easy addition or modification of scrubbers, the ability to use different acids and the ease of using wetting/emulsifying agents and indicators in the acid.
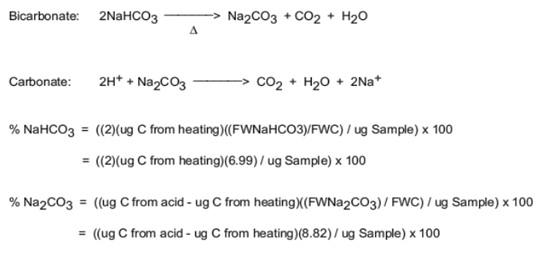
STOICHIOMETRY AND CALCULATIONS
ADDITIONAL INFORMATION
For information about the instruments capabilities for specific types of samples, or to have a sample analysis completed, contact the UIC, Inc. Applications Laboratory.